▷ K-Bio Industry in the Spotlight with the inauguration of Trump's second term
▷ TG-C, the world's first cell gene therapy for knee osteoarthritis, close to fruition
▷ Innovative new drug for osteoarthritis that provides pain relief and functional improvement for approximately two years with a single injection
▷ Anticipated entry into the osteoarthritis market, estimated to exceed 5 trillion won by 2031.
Kolon Group held a press conference at Kolon TissueGene's headquarters in Maryland, USA, on the 11th (local time) to introduce the development process and FDA clinical trial progress of TG-C, a cell gene therapy for osteoarthritis. The event was attended by Kim Seon-jin, Chief Medical Officer (CMO) of Kolon TissueGene, and Dr. Jeremy Hoff, a specialist who participated in the U.S. Phase 3 clinical trial. Following the briefing, participants toured the research facilities to observe the TG-C manufacturing process, witnessed a drug administration demonstration, and conducted interviews with relevant personnel. Kolon TissueGene is a biotech company under the Kolon Group that developed TG-C and was established in the U.S. in 1999.
At the meeting, participants discussed the global restructuring of the trade order anticipated with the launch of President Trump's second administration in January, confirmed the global competitiveness of Korean bio companies, and introduced the current status and business strategy of Kolon TG-C, which is entering the final stage of its Phase III clinical trials. Currently, the global bio market is facing various uncertainties with the launch of the new US administration. Given that three out of the 47 agenda items outlined in President Trump's campaign manifesto, Agenda47, are focused on the biotech industry, significant interest and changes are anticipated in this sector.
Trump's second term and the K-bio industry
The Trump administration is pursuing policies aimed at protecting the competitiveness of the domestic biotechnology industry while curbing the rapidly growing Chinese biosimilar (medicines that replicate expired patented drugs) business. With the passage of the Biosecurity Act in the US, access to the US market by Chinese companies will be restricted, creating opportunities for competing biotechnology companies. Notably, Kolon TissueGene, a company directly established in the U.S., is exempt from the Trump administration's trade tariff policies.
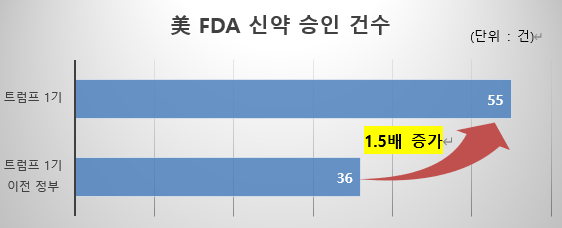
Along with this, the appointment of Marty Makary as the new FDA commissioner, who is committed to regulatory relaxation and science-based policy-making, and the expansion of the role of the Department of Government Efficiency (DOGE), led by Elon Musk and tasked with reducing the size of the US federal government, are also expected to have an impact on the biotech industry as a whole. As a result, the approval process for innovative new drugs is likely to become faster and more streamlined during the second Trump administration. For reference, during the first Trump administration, the number of new drug approvals increased by nearly 1.5 times compared to the previous administration, from an average of 36 to 55. (Source: Evaluate Pharma, a global market research firm)
The world's first cell gene therapy for osteoarthritis, Kolon's TG-C
Kolon Group established TissueGen Inc., the developer of TG-C, in the United States in 1999. They extracted cells necessary for the treatment of osteoarthritis from donated cartilage cells, cultured them, and secured the master cells that became the origin of the current TG-C. Subsequently, they began Phase 1 clinical trials in the United States in 2006, progressed to Phase 2 trials in 2010, entered Phase 3 trials in 2015, and completed the large-scale patient dosing procedure for Phase 3 trials in July 2024. The Phase 3 clinical trial of TG-C in the U.S. involved 1,066 participants, the largest scale among domestic companies, demonstrating high market interest and trust in the new drug. Going forward, Kolon TissueGene plans to undergo a two-year patient follow-up period for clinical trial participants by July 2026 and then proceed with the approval process for commercial sales in the U.S.
Kolon TissueGene's TG-C is the world's first cell-gene therapy for osteoarthritis and is an innovative new drug that patients suffering from knee osteoarthritis have never experienced before. Until now, patients suffering from pain due to cartilage wear in the knee joint had no clear treatment options other than regularly receiving painkillers or steroid injections for the knee joint, or undergoing surgical procedures to implant artificial joints. Especially given the nature of knee osteoarthritis, which affects many elderly patients, there was a high level of resistance to side effects or surgery. TG-C, as an innovative new drug, offers the potential for symptom improvement and structural joint enhancement for approximately two years with a single injection into the knee. As a cell-gene therapy, it is expected to significantly improve patients' quality of life by eliminating the side effects of painkillers or steroid injections and the burden of surgery.
TG-C consists of two components: Component 1 and Component 2. Component 1 consists of chondrocytes necessary for joints, while Component 2 contains the TGF-β1 gene, which promotes the proliferation of chondrocytes and has the ability to alleviate inflammation that causes joint pain. When Component 1 and Component 2 are mixed and injected into the knee, a single injection can provide relief from pain for approximately two years. The injection site for TG-C is limited to the joint cavity (the empty space between cartilage in the knee joint), which has no blood vessels, ensuring safety. Clinical Phase 2 results show a response rate of approximately 86%, which is significantly higher than that of competitive treatments. For reference, the response rate for rheumatoid arthritis treatments is approximately 60–70%.
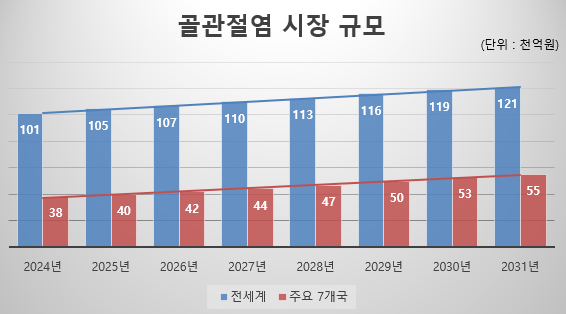
Currently, the number of osteoarthritis patients in the seven major countries (US, France, Germany, Italy, Spain, UK, and Japan) targeted by Kolon's TG-C for its initial market entry is estimated to be approximately 42 million as of 2021. Among these, patients requiring TG-C prescriptions account for 48% of the total, with North American patients accounting for approximately 44% of the total. Considering this patient distribution, the osteoarthritis treatment market focused on pain relievers in the seven major countries is estimated to be approximately 3.8 trillion won in 2024, with an annual growth rate of approximately 5.3%, and the market size in the seven major countries is expected to exceed approximately 5.5 trillion won (approximately 12 trillion won based on the global market) by 2031. (Source: GlobalData, Osteoarthritis: Epidemiology Forecast to 2031, June 17, 2022) Kolon TissueGene plans to challenge the development of TG-C into a global blockbuster new drug with annual sales exceeding 4 trillion won.
In addition to TG-C, there are several other global knee osteoarthritis treatments currently in development, but most are still in Phase II clinical trials or have completed Phase III trials without securing meaningful efficacy data and remain at a standstill. TG-C is the only one among these that has been recognized for its efficacy and safety, having completed the Phase 3 clinical trial dosing process and is now smoothly preparing for the BLA (U.S. FDA marketing authorization procedure). Among innovative new drugs in the knee osteoarthritis field, TG-C is the only one that has reached the marketing authorization procedure stage.
Unwavering challenge, TG-C
Under the group vision of “LifeStyle Innovator,” Kolon Group has been developing products and services to improve the quality of our lives in various fields, including biotechnology, chemical materials, construction, fashion distribution, and mobility. In 1999, Kolon Group pioneered the uncharted field of cell and gene therapy development, which no one else dared to attempt, and achieved scientific success through more than 20 years of continuous investment and research.
However, the process was not all smooth sailing. In 2017, the product was officially launched in Korea under the name ‘INVOSSA’ after receiving product approval, but in 2019, a cell origin error was discovered, the company voluntarily reported it and suspended sales. In the US, clinical trials were also put on hold. However, after undergoing an active defense process based on scientific data, the company received approval from the U.S. FDA in 2020 to resume Phase 3 clinical trials. Despite difficulties in proceeding with clinical trials due to the global pandemic that followed, the company successfully completed a large-scale clinical trial involving over 1,000 participants by 2024.
Currently, there are significant differences between the new drug development environments of the Korean Ministry of Food and Drug Safety (MFDS) and the US Food and Drug Administration (FDA). The MFDS is based on so-called positive regulation (which prohibits all acts except those explicitly permitted by law), while the FDA is based on negative regulation (which permits all acts except those explicitly prohibited by law). This difference was clearly evident in the Invossa incident. While the MFDS revoked INVOSSA's domestic marketing authorization within about three months, the FDA conducted scientific verification procedures for nearly a year after suspending the clinical trial. In April 2020, the FDA notified the resumption of the clinical trial and stated that the issues had been “fully satisfied.”
TG-C is also endeavoring to obtain DMOAD (disease-modifying osteoarthritis drug) qualification, which structurally inhibits the progression of arthritis and promotes recovery, thereby fundamentally treating the cause of the disease. If the DMOAD effect is recognized by the FDA, it will be recorded as the world's first case in the field of osteoarthritis treatment and is expected to bring great hope to osteoarthritis patients worldwide.
TG-C is currently expanding its treatment targets to include hip and spinal osteoarthritis in addition to knee osteoarthritis. In the hip osteoarthritis (Hip-OA) area, TG-C has been recognized for its efficacy in knee osteoarthritis, allowing it to skip Phase 1 clinical trials and proceed directly to Phase 2. The spinal area has also received approval for Phase 1 clinical trials, with plans to gradually expand TG-C's treatment targets.
<Reference>
■ Current status of INVOSSA, a product launched domestically by TG-C
While developing TG-C in the US, Kolon TissueGene has also been working hard to launch the product in South Korea. The launch process in South Korea was handled by Kolon Life Science, not Kolon TissueGene. Starting with Phase 1 clinical trials in Korea in 2006, the product received marketing authorization from the Ministry of Food and Drug Safety (MFDS) in 2017 (the first such approval for a TG-C product) and was officially launched in Korea in November of the same year. By February 2019, approximately 3,700 patients had received the treatment. In March 2019, after realizing that the cells used in the second component were derived from kidney cells rather than cartilage cells, a voluntary sales suspension was implemented. In July 2019, the MFDS revoked the domestic marketing authorization for Invossa. As of 2025, there have been no serious adverse effects associated with Invossa.
Criminal trials and administrative proceedings are currently underway on the grounds of errors in the cell origin of Invossa. Of particular note is the result of the first criminal trial (2020go-hap-53) in November last year, which resulted in a full acquittal after four years and four months of controversy over the ingredients of Invossa. The court stated in its ruling, “While the United States resumed clinical trials after scientific review of the component error in Invossa, South Korea has continued legal disputes for years. This raises the question of how judicial control over scientific fields should be conducted.” All defendants were acquitted.
KOLON Group's mobile web is optimized for vertical screens.




